Omalizumab for angioedema
Last updated Jan. 30, 2025, by Marisa Wexler, MS

What is omalizumab for angioedema?
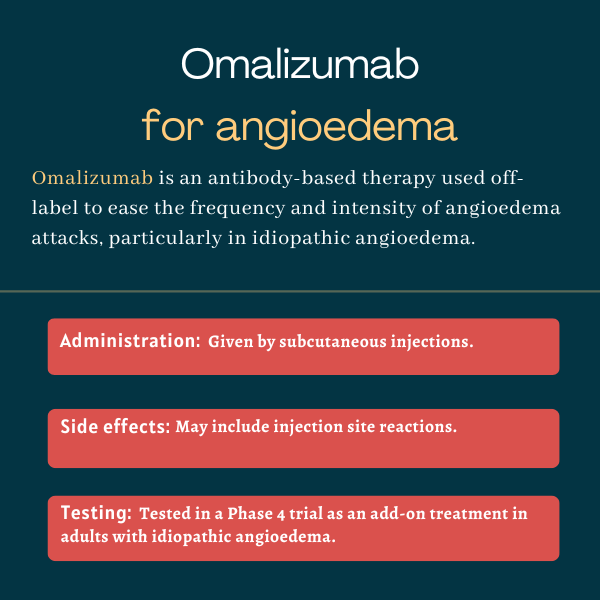
Omalizumab is an antibody-based therapy that’s used to manage some types of allergy-related conditions. It is not approved for the treatment of angioedema, but it may be used off-label in some patients, particularly individuals with idiopathic angioedema, or those whose swelling attacks lack a clear cause.
Brand names and formulations
In the U.S., omalizumab is jointly marketed by Novartis and Genentech under the brand name Xolair. The therapy is approved in that country to treat four allergy-related conditions:
- chronic rhinosinusitis (nose and sinus inflammation) with nasal polyps in adults who don’t respond to first-line treatment with nasal corticosteroids
- persistent asthma that doesn’t respond well to treatment with inhaled corticosteroids in certain patients ages 6 and older
- some types of food allergies in patients ages 1 and older
- chronic spontaneous urticaria (CSU), a condition characterized by recurring hives, in certain patients ages 12 and older.
Xolair, first approved in the U.S. in June 2003 for the treatment of moderate to severe asthma, is administered via a subcutaneous, or under-the-skin, injection. The therapy is approved for similar indications in other countries and regions worldwide.
Therapy snapshot
| Treatment name: | Omalizumab |
| Administration: | Subcutaneous injection |
| Clinical testing: | A small Phase 4 clinical trial evaluated the effects of omalizumab in idiopathic angioedema patients |
How does omalizumab work in angioedema?
Omalizumab is a monoclonal antibody that’s designed to bind and neutralize the activity of a type of antibody called immunoglobulin E, or IgE. While these antibodies are important for helping to defend the body against infections, they are also known to play a key role in allergic reactions, in which the body’s immune system overreacts to a harmless substance such as pollen.
In idiopathic angioedema, by definition, the cause of recurrent swelling is not known with certainty. But it’s thought that, at least in some patients, swelling might be driven by allergic mechanisms involving IgE — even if the specific allergen, or allergy-triggering agent, cannot be identified. As such, by neutralizing IgEs, omalizumab may help to ease swelling in these individuals.
How is omalizumab administered in angioedema?
Omalizumab is supplied as a solution in a prefilled single-dose syringe, or in a prefilled single-dose autoinjector, at three different strengths (75 mg/0.5 mL, 150 mg/mL, and 300 mg/2 mL). It is also available as a powder in single dose vials containing 150 mg of the therapy’s active ingredient that must be diluted in sterile water before being administered.
Treatment is given by subcutaneous injection, and it should be administered under the guidance of a healthcare professional. Omalizumab can be self-administered in some cases. The exact dose and frequency of administration vary depending on the specific indication for which the therapy is being used.
Because omalizumab is not formally approved for angioedema, there is no standardized dosing regimen for its use in people with the condition. However, in a Phase 4 clinical trial, patients with idiopathic angioedema were given omalizumab at a dose of 300 mg every four weeks, following the same dosing regimen that’s recommended for patients with CSU.
Omalizumab in angioedema clinical trials
Some clinical trials and case reports have described the use of omalizumab in patients with angioedema.
Data from Phase 3 trials in CSU patients, as many as 53% of whom also had angioedema, indicated that omalizumab could help ease angioedema symptoms. Some case reports also describe positive effects of the therapy’s use exclusively in patients with idiopathic angioedema.
The University of Wisconsin at Madison sponsored a small, randomized, double-blind, and placebo-controlled Phase 4 trial clinical (NCT02966314) to evaluate omalizumab in people with idiopathic angioedema. The study enrolled 10 adults who had experienced at least two attacks of idiopathic angioedema in the prior six months. Five were given monthly subcutaneous injections of omalizumab (300 mg), while the other five received placebo injections, all given on top of standard therapy, for about six months.
The trial results showed that, compared with patients on the placebo, those treated with omalizumab had significantly fewer and shorter swelling episodes. Specifically, while patients on the placebo experienced a total of 56 angioedema episodes over the course of treatment, each lasting more than five hours, those on omalizumab had seven episodes that each lasted slightly more than one hour.
Four of the five patients given omalizumab were deemed to be in remission following treatment. Omalizumab-treated patients also showed significant improvements in standardized measures of symptom severity and life quality.
The therapy’s safety profile in the Phase 4 trial was identical to that observed in previous studies involving patients with asthma and CSU. No serious side effects or deaths were reported.
Common side effects of omalizumab
The specific safety profile of omalizumab in people with angioedema is not fully known, but a delayed injection site reaction has been attributed to the therapy in a patient with idiopathic angioedema who received the medication in a Phase 4 clinical trial.
In its approved indications, common side effects of omalizumab include:
- injection site reactions, such as pain, swelling, itching, or skin discoloration
- joint pain, known as arthralgia
- fever
- cold or flu-like symptoms
- abdominal pain
- upper respiratory infection
- headache
- dizziness
- sore throat, known as pharyngitis
- ear infection, called otitis
- sinus infection, or sinusitis.
Allergic reactions
Omalizumab’s prescribing information carries a boxed warning noting that it can cause severe, life-threatening allergic reactions, known as anaphylaxis. Such reactions typically develop within an hour or two of the therapy being administered. Symptoms of anaphylaxis can include difficulty breathing, low blood pressure, fainting, hives, and swelling affecting the throat and tongue.
Due to these risks, omalizumab should only be administered in a healthcare setting equipped to manage anaphylaxis, and patients receiving the medication should be monitored for an adequate period of time following dosing. If symptoms of anaphylaxis arise, patients should seek immediate medical care.
Treatment with omalizumab should be discontinued in patients who experience a severe allergic reaction.
Risk of cancer and parasitic infections
The use of omalizumab has been linked to a slight increase in the risk of some types of cancer in clinical studies, although other studies haven’t found consistent results.
Treatment with omalizumab also may increase the risk of certain parasitic infections, such as roundworm and hookworm. Patients at risk of encountering these parasites should be carefully monitored while on omalizumab.
Inflammatory and systemic reactions
Rare cases have been reported of asthma patients on omalizumab developing heart complications, rash, and an inflammatory condition called eosinophilia, which can cause nerve damage. Omalizumab treatment should be stopped if patients show signs of eosinophilia.
There also have been reports of patients on omalizumab who have developed a combination of symptoms, including joint pain, rash, fever, and enlarged lymph nodes. Treatment should be stopped if patients display this constellation of symptoms.
Use in pregnancy and breastfeeding
A registry study did not find evidence of an increased risk of major birth defects or miscarriage when omalizumab was used during pregnancy. An association was found between omalizumab and lower birth weight, though it wasn’t clear if this association was due to the therapy itself, or because women treated with omalizumab tended to have more severe asthma than those not on the medication. Animal reproduction studies also found no evidence indicating that omalizumab might be harmful to a developing fetus.
Solid data indicating omalizumab’s presence in breast milk are lacking. However, given the fact that omalizumab is a monoclonal antibody, it’s expected that small amounts of the therapy would be present in breast milk. Most infants in the pregnancy registry study were breastfed, and the findings did not reveal any notable increase in the frequency of infections among infants breastfed by women on omalizumab compared with those not exposed to the medication or not breastfed.
A decision on whether to use omalizumab in these situations should always be made in consultation between patients and their healthcare providers, by carefully weighing the potential benefits and risks for the mother and the infant.
Angioedema News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Ekterly use urged for kids with HAE 12 and older in new international guideline
- Why it’s important to maintain your own personal health information, part 1
- HAE caregiving takes heavy emotional, personal toll: Multinational survey
- Having HAE takes toll on life quality, regardless of race, ethnicity
- Biocryst to present new Orladeyo, navenibart data at AAAAI meeting