Orladeyo (berotralstat) for HAE
What is Orladeyo for hereditary angioedema?
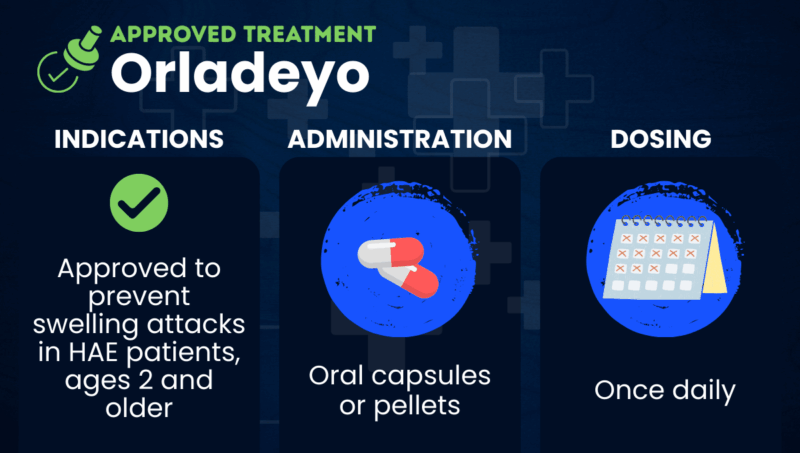
Orladeyo (berotralstat) is an approved oral therapy used for the prevention of swelling attacks in children and adults with hereditary angioedema (HAE).
In HAE, the overproduction of a molecule called bradykinin leads to sudden swelling attacks that can occur anywhere on the body.
Taken in the form of daily oral capsules or pellets, Orladeyo works by suppressing the activity of kallikrein, an enzyme needed to generate bradykinin from its precursor molecules. By inhibiting kallikrein, the medication lowers bradykinin production, thereby preventing swelling attacks.
Therapy snapshot
| Brand name | Orladeyo |
| Chemical name | Berotralstat |
| Usage | Used to prevent swelling attacks in children and adults with hereditary angioedema |
| Administration | Oral capsules or pellets |
Who can take Orladeyo?
In the U.S., Orladeyo is approved as a prophylactic therapy to prevent HAE attacks in adults and children, ages 2 years and older.
There are no contraindications for its use. However, the medication should not be used for the on-demand treatment of acute HAE attacks. Orladeyo should also be avoided in children younger than 12 who have severe kidney impairments or moderate-to-severe liver impairments, and in all patients with end-stage kidney disease.
Orladeyo is approved for HAE in more than 45 countries globally, including the European Union and Canada, although approved age ranges vary.
How is Orladeyo administered?
For adult and pediatric patients ages 12 years and older, Orladeyo is available in the form of oral capsules, taken once daily with food. The recommended dose is 150 mg.
For children ages 2-11, Orladeyo is available in an oral pellet (granule) formulation that’s taken with food once daily at a weight-based dose. The pellets should never be chewed or crushed, and can be taken in either of two ways:
- poured directly into the mouth and swallowed immediately with a non-acidic liquid such as water or milk
- sprinkled over one tablespoon of non-acidic soft food (e.g., mashed potatoes, creamed corn, puréed peas, bananas, or carrots) and consumed within 10 minutes.
Dose modifications may be recommended for certain patients with liver impairments or those who experience persistent gastrointestinal side effects from the medication.
Orladeyo in clinical trials
Orladeyo’s approvals for adults and children were largely supported by two Phase 3 clinical trials:
- The placebo-controlled APeX-2 trial (NCT03485911) involved adults and adolescents with HAE, ages 12 and older. Results showed that Orladeyo significantly reduced the frequency of swelling attacks compared to a placebo over about six months, with benefits sustained for nearly two years. Quality-of-life improvements were also observed.
- The open-label APeX-P trial (NCT05453968) involved children with HAE, ages 2-11. Interim data showed the therapy led to early and sustained reductions in attack rates, which were sustained for nearly a year, with a safety profile similar to that observed in older patients.
Orladeyo side effects
The most common side effects of Orladeyo include:
- abdominal pain
- vomiting
- diarrhea
- back pain
- acid reflux
Orladeyo can cause heart rhythm abnormalities if taken at higher-than-recommended dosages. It may also interact with some other medications. Patients should always take Orladeyo exactly as prescribed by their doctor and inform their healthcare provider about all other medications they are using.
Angioedema News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by