Firazyr (icatibant) for hereditary angioedema
Last updated Feb. 14, 2024, by Marta Figueiredo, PhD

What is Firazyr for hereditary angioedema?
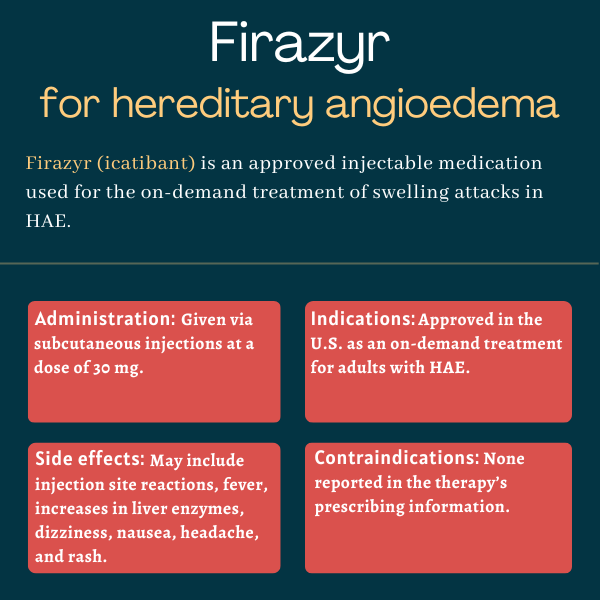
Firazyr (icatibant) is an under-the-skin, or subcutaneous, injectable therapy approved for on-demand management of swelling attacks in people with hereditary angioedema (HAE). It is available for adults with HAE in the U.S., as well as for patients ages 2 and older in Canada and Europe.
The therapy originally was developed by Jerini AG, which was acquired in 2008 by Shire, which, in turn, is now part of Takeda Pharmaceuticals.
Several generic versions of Firazyr also are available in the U.S., including those marketed by Cipla and Eugia Pharma Specialities.
Therapy snapshot
| Brand name: | Firazyr |
| Chemical name: | Icatibant |
| Usage: | On-demand treatment of swelling attacks in HAE patients |
| Administration: | Subcutaneous injection |
How does Firazyr work?
All types of HAE are characterized by sudden swelling attacks. These are driven by excessive levels of bradykinin, a pro-inflammatory molecule that promotes blood vessel dilation and leakage in the deep layers of the skin, resulting in swelling and pain.
People with the most common forms of HAE — types 1 and 2 — have genetic mutations that lead to a deficiency in C1 inhibitor (C1-INH), a protein that blocks the activity of kallikrein — the enzyme that controls bradykinin production.
Firazyr is a lab-made molecule that works by selectively binding to and blocking the bradykinin receptor B2 protein. This ultimately prevents bradykinin from binding to the receptor B2 protein and subsequently promoting its inflammatory and blood vessel leakage effects. As such, the therapy is expected to relieve symptoms of the potentially serious and painful swelling attacks that mark HAE.
Who can take Firazyr?
Firazyr was approved by the U.S. Food and Drug Administration in August 2011 for the treatment of acute swelling attacks in adults with HAE. That decision made Firazyr the third medication to be approved in the U.S. for HAE, but the first to allow self-administration through subcutaneous injections upon signs of an attack.
The therapy also has been available in Europe, since July 2008, for adult HAE patients with C1-INH deficiency. In October 2017, the European Commission expanded Firazyr’s use to children ages 2 and older with HAE and C1-INH deficiency, making it the first subcutaneous treatment in Europe for HAE attacks in such a young patient population.
Firazyr also is approved in Canada for adults, adolescents, and children ages 2 years and older with HAE and C1-INH deficiency.
Who should not take Firazyr?
The prescribing information for Firazyr lists no contraindications to its use.
How is Firazyr administered?
Firazyr is available as a single-use, prefilled syringe containing 30 mg of the medication’s active ingredient in 3 mL of a clear and colorless liquid.
The recommended dose of Firazyr for adults is 30 mg administered via a single subcutaneous injection in the abdominal area upon recognition of HAE attack symptoms. If patients continue to experience symptoms or the initial symptoms return, additional doses, separated by no less than six hours, may be given. However, patients should not receive more than three doses of the medication in a period of 24 hours.
The therapy can be self-administered by patients who have been trained by a healthcare professional in how to perform injections.
After confirming that the Firazyr solution does not contain any particles, is not cloudy, and does not have an unusual color, patients should attach the needle to the syringe. Both are provided in the medication carton.
Before administration, the injection site — which should not have scars, or be bruised, swollen, or painful — should be disinfected. Firazyr should be administered for at least 30 seconds. Additional information on how to administer the therapy can be found on Firazyr’s website, but this does not replace initial training by a healthcare professional.
Firazyr in clinical trials
Firazyr’s approval in the U.S. was mainly supported by positive data from three Phase 3 clinical trials: FAST-1 (NCT00097695), FAST-2 (NCT00500656), and FAST-3 (NCT00912093).
These studies evaluated the safety and efficacy of the now-approved 30 mg dose of Firazyr. It was tested against a placebo in FAST-1 and FAST-3, and versus oral tranexamic acid in FAST-2, to treat swelling attacks in more than 200 adults with HAE type 1 or 2.
Oral tranexamic acid had been previously reported to reduce HAE attack symptoms, but its efficacy had not been proved in controlled trials.
FAST-1 and FAST-2
The FAST-1 study enrolled 84 patients at a single site in the U.S. Among them, 56 were randomly assigned to receive a subcutaneous injection of either Firazyr or a placebo upon experiencing signs of a skin and/or abdominal swelling attack.
FAST-2 recruited 85 patients at a single Italian site. A total of 74 participants underwent randomization to take either Firazyr or tranexamic acid, at a daily dose of 3 g for two days, in the event of moderate to very severe symptoms of a skin and/or abdominal attack.
In both trials, the main goal was to assess the median time to clinically significant symptom relief, as indicated by a 30% reduction in a visual-analogue scale (VAS) of symptom severity relative to attack onset.
The results showed that the main goal was met after 2.5 hours with Firazyr versus 4.6 hours with a placebo in the FAST-1 trial. In FAST-2, the main goal was met in two hours with Firazyr versus 12 hours with tranexamic acid. Group differences reached statistical significance only in the FAST-2 study.
Still, in both trials, patients on Firazyr achieved nearly complete symptom resolution significantly faster than those on a placebo (8.5 vs. 19.4 hours) or on tranexamic acid (10 vs. 51 hours). Firazyr’s superiority also was observed for median time to first symptom lessening in both studies.
In addition, a greater proportion of Firazyr-treated patients achieved clinically significant symptom relief after four hours in both FAST-1 (67% vs. 46%) and FAST-2 (80% vs. 31%).
Those on Firazyr also were less likely to use rescue medication within the first 48 hours than those on a placebo (22% vs. 52%) or on tranexamic acid (17% vs. 29%).
FAST-3
The FAST-3 study enrolled 98 patients at sites in the U.S. and other countries. Those experiencing moderate to severe symptoms of skin and/or abdominal attacks, or mild to moderate symptoms of laryngeal attacks, were randomly assigned to receive either Firazyr or a placebo.
Laryngeal attacks are those involving the larynx, or voice box. They can be life-threatening as they can lead to airway obstruction and asphyxiation.
The trial’s main goal was assessing the median time to a sustained 50% drop in attack symptom severity based on composite VAS scores.
For nonlaryngeal attacks, the results indicated that Firazyr was significantly faster than a placebo at reducing symptom severity by half (two vs. 19.8 hours). Also, onset of primary symptom relief was achieved significantly earlier by Firazyr-treated patients than by those on a placebo (1.5 vs 18.5 hours), meeting a key secondary goal.
The therapy also was associated with a significantly faster initial symptom relief, based on both patients’ self-reports and investigators’ assessments (0.8 vs. about 3.5 hours), and complete symptom resolution (eight vs. 36 hours).
For the five patients experiencing mild to moderate laryngeal attacks, median time to 50% reduction in symptom severity was 2.5 hours with Firazyr and 3.2 hours with a placebo. Both patients assigned to the placebo went on to receive the therapy.
No Firazyr-treated patients required rescue medication before achieving symptom relief for nonlaryngeal attacks compared with 36% of those given a placebo. Moreover, significantly fewer patients on the therapy needed rescue medication at any time during the attack (7% vs. 40%).
Pooled trial analysis
In all three trials, any patient having a subsequent attack of sufficient severity to necessitate treatment was included in each study’s open-label extension phase and received Firazyr.
A total of 987 HAE attacks in 225 patients were treated with 1,076 doses of Firazyr in these trials. The median times to a 50% reduction in a composite VAS score were similar between the first five treated attacks, ranging from 1.5 to 2.4 hours. And most of these attacks (93%) were treated with a single Firazyr dose.
Patients experiencing laryngeal symptoms as the first attack in FAST-1 and FAST-2 also were treated with Firazyr in the respective extension phases. Meanwhile, FAST-3 participants with severe laryngeal symptoms were given Firazyr in its extension phase.
In total, 60 trial participants were treated with Firazyr for laryngeal attacks. The therapy’s efficacy was generally similar to that reported for non-laryngeal attacks. Pooled patient-reported data showed that initial symptom improvement occurred after a median of just longer than a half-hour, with times ranging between 0.6 to 0.8 hours across the three studies.
FAST-4
A Phase 3b trial called FAST-4 (NCT00997204) evaluated the safety, convenience, and effectiveness of self-administered Firazyr in 97 HAE patients experiencing swelling attacks deemed by the patient to require treatment.
Data demonstrated that a 30% reduction in the main symptom occurred after a median of two hours, and a 50% reduction in symptom severity was noted after a median of 3.8 hours; both were assessed through VAS-based scoring. Three patients self-administered the therapy for laryngeal attacks, and all considered that these were satisfactorily resolved by 48 hours, or two days.
A single Firazyr injection was enough to resolve the attack in most patient (91.8%), and two patients used rescue mediation for worsening or recurrent symptoms. Participants also reported a high degree of satisfaction, convenience, and ease of use with Firazyr self-administration.
Trials in children and adolescents
An international Phase 3 trial (NCT01386658) evaluated Firazyr’s safety, pharmacological properties, and efficacy in 32 children and adolescents, ages 2 to 17, with HAE type 1 or 2, who experienced skin and/or abdominal attacks.
A single weight-based dose of Firazyr (0.4 mg/kg; maximum 30 mg) was generally well-tolerated and resulted in a 20% reduction in symptom severity after a median of one hour, as assessed with a composite symptom score, the results showed. None of the patients needed rescue medications within 48 hours of Firazyr administration.
Another Phase 3 trial (NCT04654351) tested the therapy in two Japanese HAE type 1 or 2 pediatric patients, ages 2-17, who weighed a minimum of 12 kg (about 26 pounds).
The results showed that a single Firazyr injection, at a weight-based dose, rapidly eased symptoms of four skin and/or abdominal attacks, with patients achieving symptom relief after about one hour.
None needed rescue medications for their attacks. The therapy’s safety and pharmacological profiles also were consistent with those reported for adults and other pediatric patients.
Common side effects of Firazyr
The most common side effects of Firazyr are injection site reactions, including redness, bruising, swelling, burning, itching, and pain. Other common side effects that may occur with the therapy include:
- fever
- high levels of the transaminase enzyme, indicating potential liver damage
- dizziness
- rash
- nausea
- headache.
Laryngeal attacks
Given that laryngeal HAE attacks can potentially cause airway obstruction, patients experiencing such an attack should self-inject Firazyr and seek immediate medical attention.
Use in pregnancy and breastfeeding
Firazyr has not been appropriately evaluated in pregnant or nursing women. Available real-world data from pregnant women on the therapy suggest that Firazyr may not be associated with adverse maternal or fetal outcomes. But studies in pregnant rats and rabbits indicated a potential risk of harm to the fetus.
Patients who are pregnant or planning to become pregnant should discuss with their healthcare team the potential benefits and risks of using Firazyr in these circumstances.
It is not known if Firazyr can be found in human breast milk and whether it has any impact on nursing infants or on milk production. However, the medication was detected in the milk of lactating rats treated with Firazyr, suggesting that the same may happen in human patients.
As such, the benefits of breastfeeding should be considered along with the mother’s need for Firazyr and the potential adverse effects to the nursing infant.
Angioedema News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- New study explores how adults with HAE recognize and manage feelings
- Sometimes, even our best efforts aren’t enough
- Ekterly use urged for kids with HAE 12 and older in new international guideline
- Why it’s important to maintain your own personal health information, part 1
- HAE caregiving takes heavy emotional, personal toll: Multinational survey